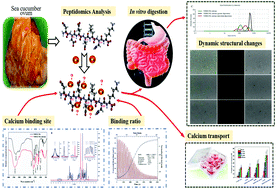
In this study, a novel calcium-binding heptapeptide (NDEELNK) that is released during the trypsin hydrolysis of sea cucumber ovum was identified by peptidomics. The calcium binding mode, in vitro digestion profile and calcium absorption of the NDEELNK–calcium complex were investigated. The NDEELNK peptide could spontaneously bind calcium with a 1 : 1 stoichiometry, and the calcium-binding site might involve the carboxyl oxygen and amino nitrogen atoms of two glutamic acid and one aspartic acid residues in the NDEELNK peptide. The NDEELNK–calcium complex underwent disaggregation and self-aggregation in a mesh of smaller size during simulated gastrointestinal digestion, clarified by dynamic light scattering and confocal laser-scanning microscopy. In addition, the NDEELNK–calcium complex could be conducive to calcium absorption across Caco-2 cell monolayers. The findings from this research suggest possible utilization of hydrolyzed peptides from sea cucumber ovum as dietary supplements to improve calcium absorption.