Preparation and identification of novel inhibitory angiotensin-I-converting enzyme peptides from tilapia skin gelatin hydrolysates: inhibition kinetics and molecular docking
Abstract
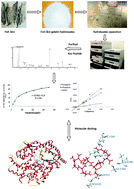
Tilapia skin gelatin was hydrolyzed by successive simulated gastrointestinal digestion, and the hydrolysates were further separated by transport across a Caco-2 cell monolayer. Angiotensin-I-converting enzyme inhibitory (ACEI) peptides were separated by successive chromatographic steps from the transport hydrolysates. We have identified two key ACEI peptides, namely VGLPNSR (741.4133 Da) and QAGLSPVR (826.4661 Da) with IC50 values of ACEI activity of 80.90 and 68.35 μM, respectively. Lineweaver–Burk plots indicated that the inhibitory ACE kinetics of the two peptides were noncompetitive. Molecular docking simulation showed that the two peptides could interact with the ACE site via hydrogen bonds with high binding power. However, the hydrogen bonds were not formed with the key amino acid residues in the active site of ACE. This finding was in accordance with the noncompetitive inhibition. This study established a novel approach to identify key ACEI peptides and suggested the use of tilapia peptides as functional food ingredients to prevent hypertension.


 Please wait while we load your content...
Please wait while we load your content...