Antioxidant activity and inhibitory effects of 2-hydroxy-3-methylcyclopent-2-enone isolated from ribose–histidine Maillard reaction products on aldose reductase and tyrosinase†
Abstract
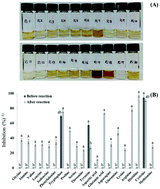
This study aimed to better understand the functional properties of ribose and 20 amino acid Maillard reaction products (MRPs). The ABTS+ radical scavenging ability of the ribose–20 amino acid MRPs was evaluated. Among the MRPs, ribose–histidine MRPs (RH-MRPs) showed the highest inhibitory activities on the ABTS+ radical scavenging ability, aldose reductase (AR), and tyrosinase compared to other MRPs. Functional compounds with antioxidant and AR inhibitory activities have been recognized as an important strategy in the prevention and treatment of diabetic complications, and the search for tyrosinase inhibitors is important for the treatment of hyperpigmentation, development of skin-whitening agents, and use as preservatives in the food industry. On this basis, we sought to isolate and identify compounds with inhibitory activities against AR and tyrosinase. RH-MRPs were heated at 120 °C for 2 h and fractionated using four solvents: methylene chloride (MC), ethyl acetate, n-butanol, and water. The highest inhibitions were found in the MC fraction. The two compounds from this fraction were purified by silica gel column and preparative thin layer chromatography, and identified as 2-hydroxy-3-methylcyclopent-2-enone and furan-3-carboxylic acid. AR inhibition, tyrosinase inhibition, and ABTS+ scavenging (IC50) of 2-hydroxy-3-methylcyclopent-2-enone were 4.47, 721.91 and 9.81 μg mL−1, respectively. In this study, inhibitory effects of 2-hydroxy-3-methylcyclopent-2-enone isolated from RH-MRP were demonstrated on AR, tyrosinase, and its antioxidant activity for the first time. RH-MRP and its constituents can be developed as beneficial functional food sources and cosmetic materials and should be investigated further as potential functional food sources.

 Please wait while we load your content...
Please wait while we load your content...