Model potential study of non-valence correlation-bound anions of (C60)n clusters: the role of electric field-induced charge transfer†
Abstract
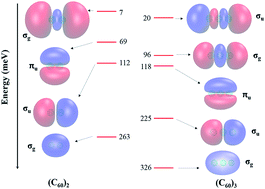
A polarization model which accounts for electric field-induced charge transfer between fullerene molecules is introduced. Application of this model to the C60 dimer and trimer shows that intermolecular charge transfer makes a significant contribution to the polarizabilities of these clusters. This polarization model is incorporated into a one-electron Hamiltonian for describing non-valence correlation-bound anions, allowing us to further demonstrate that intermolecular charge transfer also results in increased stability of these anion states.
- This article is part of the themed collection: Advances in ion spectroscopy - from astrophysics to biology


 Please wait while we load your content...
Please wait while we load your content...
