Selectivity and polarization in water channel membranes: lessons learned from polymeric membranes and CNTs
Abstract
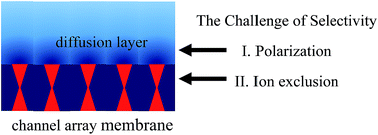
Water channels are employed by nature to move pure water across cell membranes while selectively rejecting salts. At present, synthetic channels successfully mimic water permeation, yet even the best channels, such as carbon nanotubes (CNTs) and graphene oxide stacks, still fall short of the selectivity target. The present paper analyzes factors that may help to enhance and control salt rejection based on the lessons learned from conventional membranes and CNTs. First, it highlights the importance of raising the ion self-energy (dielectric mechanism), which suggests that having the channels both narrow and surrounded by a low-dielectric environment is key to high selectivity. In contrast, pore charge alone is insufficient, yet it may help to enhance and tune ion rejection, provided that non-mean-field effects enhanced in low-dielectric pores, such as ion association and sorption, especially of H+ and OH− ions, are properly understood and addressed in the channel design. Second, the role of concentration polarization (CP) is analyzed, which shows that the CP level is apparently low in isolated channels or microscopically small membranes. However, the geometry of the diffusion field should change and CP should increase drastically in macroscopic membranes incorporating densely spaced channel arrays. If not properly addressed in membrane design, the increased CP level in scaled-up channel-based membranes may significantly compromise the observed selectivity and require that target of selectivity be re-set to an even more challenging value. These points may help guide the future development of high-performance artificial water channels and their scale-up towards utilization in next-generation water purification membranes.
- This article is part of the themed collection: Artificial Water Channels


 Please wait while we load your content...
Please wait while we load your content...