Molecular scale structure and dynamics at an ionic liquid/electrode interface†
Abstract
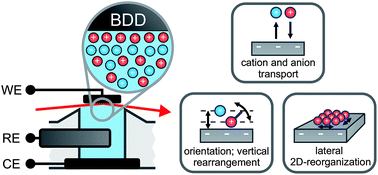
After a century of research, the potential-dependent ion distribution at electrode/electrolyte interfaces is still under debate. In particular for solvent-free electrolytes such as room-temperature ionic liquids, classical theories for the electrical double layer are not applicable. Using a combination of in situ high-energy X-ray reflectivity and impedance spectroscopy measurements, we determined this distribution with sub-molecular resolution. We find oscillatory charge density profiles consisting of alternating anion- and cation-enriched layers at both cathodic and anodic potentials. This structure is shown to arise from the same ion–ion correlations dominating the liquid bulk structure. The relaxation dynamics of the interfacial structure upon charging/discharging were studied by impedance spectroscopy and time resolved X-ray reflectivity experiments with sub-millisecond resolution. The analysis revealed three relaxation processes of vastly different characteristic time scales: a 2 ms scale interface-normal ion transport, a 100 ms scale molecular reorientation, and a minute scale lateral ordering within the first layer.
- This article is part of the themed collection: Ionic liquids: from fundamental properties to practical applications


 Please wait while we load your content...
Please wait while we load your content...