Using single-particle ICP-MS for monitoring metal-containing particles in tap water†
Abstract
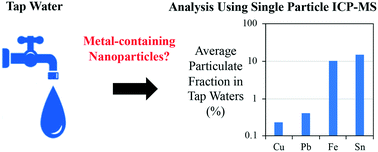
Engineered, natural or incidental colloidal-sized materials in tap water may originate from source water or be generated in distribution systems (e.g., corrosion-related). An optimized single-particle (sp)-ICP-MS technique was applied to tap water samples (n = 50) collected from three buildings to analyze for Pb, Fe, Sn, Cu, Ag and Ti-containing particles. The Pb, Sn and Fe-containing particles were detected at an average concentration (ng L−1) of 1.2 (range: 0.06–4.8), 1.8 (range: 0.11–14), and 88 (range: 26–890), respectively, representing the corresponding total dissolved metal concentrations at a minimum of 0.4%, 10%, and 15%. No particulate Ti and Ag were observed in the samples. The Pb concentrations in the first 125 mL fraction collected were on average three times higher than those in the subsequent samples. Detection of the Cu particles required modification of the sample introduction system (direct self-aspiration into the nebulizer) to reduce matrix interaction with the auto-sampler tubing. The Cu particles were detected in 50% of the analyzed samples at an average concentration of 69 (range: 15–136) ng L−1. While all the metal concentrations were below the health advisory levels, this study showcases the feasibility and first application of spICP-MS to monitor metal-containing particles in tap waters, and the results suggest that the particulate forms of the studied elements may represent a significant fraction of the bulk elemental concentration in tap water.


 Please wait while we load your content...
Please wait while we load your content...