Integrated, multi-process approach to total nutrient recovery from stored urine†
Abstract
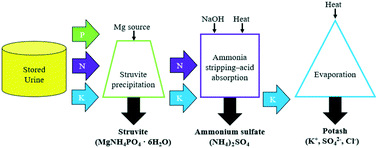
This study investigated an integrated, multi-process approach of using struvite precipitation, ammonia stripping–acid absorption, and evaporation to recover phosphorus (P), nitrogen (N), and potassium (K), respectively, from stored urine. The process produces separate nutrient products that can then be recombined to produce customized fertilizers of any NPK ratio. Bench-scale experiments were conducted using three stored urine solutions: synthetic urine, synthetic urine with six endogenous metabolites, and real urine. For struvite precipitation, MgCl2·6H2O, MgCO3, and MgO were tested and dosed at a molar ratio of 1.1 : 1 Mg : P. There was a statistically significant difference between total phosphate (TP) recovered by each magnesium (Mg) source and urine solution; MgCl2·6H2O (91–94% TP recovered) > MgCO3 (55–77%) > MgO (52–66%) and real urine > synthetic urine with six endogenous metabolites > synthetic urine. For ammonia stripping–acid absorption, there was a statistically significant difference between TAN recovery and experimental stripping conditions where increasing both the pH and temperature recovered a higher percent of TAN compared to solely increasing the pH or temperature of the solution. In real urine, consumed cost for stripping increased as follows: control condition of pH 9.2, 22 °C < elevated pH condition of pH 10.5, 22 °C < elevated temperature condition of pH 9.2, 70 °C. There was no statistically significant difference between the Mg source and TAN recovery in real urine and synthetic urine with metabolites but there was in synthetic urine. Furthermore, the amount of TAN recovered in real urine and synthetic urine with metabolites was consistently greater than or approximately equal to synthetic urine. This suggests that using synthetic urine as a proxy for real urine is not suitable for N recovery. For evaporation, there was a statistically significant difference between the urine solution and conditions for N recovery (i.e., temperature and/or pH) on K recovery and product purity. As the pH was increased, the purity of the final K product, potash, decreased due to sodium from NaOH. Results from this study show that an integrated, multi-process approach to urine treatment can achieve approximately 99% N, 91% P, and 80% K recovery as fertilizer products.


 Please wait while we load your content...
Please wait while we load your content...