Role of tertiary amines in enhancing trihalomethane and haloacetic acid formation during chlorination of aromatic compounds and a natural organic matter extract†
Abstract
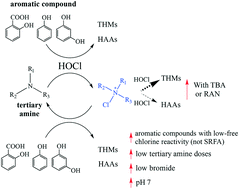
Tertiary amines are prevalent in waters due to anthropogenic inputs and are known to enhance organic compound degradation while increasing disinfection by-product (DBP) formation, via the strong chlorinating agent, R3N–Cl+. This study explored how tertiary amines can enhance trihalomethane (THM) and haloacetic acid (HAA) formation when various aromatic compounds (salicylic acid (SA), phenol (PHE) and resorcinol (RES)) and Suwannee River fulvic acid (SRFA) are chlorinated. Four tertiary amines (2-(N-morpholino)ethanesulfonic acid (MES), tributylamine (TBA), trimethylamine (TMA), and ranitidine (RAN)) were tested. In synthetic solutions, chloroform (CHCl3) and trichloroacetic acid (TCAA) were enhanced by up to 20× with tertiary amines, which also occurred at low amine doses ([tertiary amine]0 = 0.5 × [aromatic compound]0). Enhancement decreased with aromatic compound type where SA > PHE > or ≈ RES for CHCl3 and SA > PHE for TCAA. However, TBA and RAN generated up to 2.0 and 7.9% molar yields of TCAA and CHCl3, respectively, with free chlorine alone. With MES, enhancement was maximized at pH 7 while brominated THMs and HAAs were not enhanced upon bromide addition. Thus, THMs and HAAs were predicted to be enhanced via an aromatic compound reaction with R3N–Cl+ when not outcompeted by aromatic compound or tertiary amine reactions with free chlorine alone. SRFA exhibited no or limited CHCl3 and TCAA enhancement with MES, respectively, likely due to the presence of aromatic functional groups that strongly reacted with free chlorine alone. Overall, waters with low tertiary amine and bromide concentrations can potentially enhance THM and HAA formation when aromatic functional groups with low free chlorine reactivity are chlorinated.


 Please wait while we load your content...
Please wait while we load your content...