Surface defects enhance the adsorption affinity and selectivity of Mg(OH)2 towards As(v) and Cr(vi) oxyanions: a combined theoretical and experimental study†
Abstract
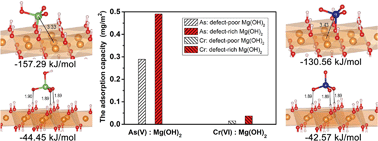
The surface defects of adsorbents are generally considered to be the active sites for adsorbates, and the adsorption behaviors of a variety of pollutants in wastewater are strongly influenced by the surface defects of the adsorbents. With simple chemical and structural properties, nano-Mg(OH)2 exhibits a high adsorption affinity and selectivity to heavy metals from wastewater. The insight into the surface complex structures of heavy metals on the Mg(OH)2 surface is fundamental for both the mechanism and application of defective adsorbents in wastewater treatment. In this study, the impact of surface defects of Mg(OH)2 nanoplates on the structure, energy, and adsorption amounts of toxic heavy metal oxyanions (HAsO42− and CrO42−) was investigated by combining the first-principles calculations and batch experiments. The first-principles calculations illustrated that the heavy metal oxyanions can be steadily incorporated into the surface defects to form inner-sphere complexes. The incorporated heavy metal oxyanions have strong charge–charge and chemical bonding interactions with the surface defect sites, which are much stronger than the hydrogen bonding interactions with the surface OH sites. Moreover, the adsorption affinity of surface defects towards heavy metal oxyanions is stronger than that towards other oxyanions (e.g. SO42− and NO3−), indicating the high adsorption selectivity of heavy metal oxyanions over other oxyanions. Batch adsorption experiments verified that the adsorption of defect-rich Mg(OH)2 adsorbents towards heavy metal ions, e.g., As(V) and Cr(VI), was favorable even under the condition of low concentration (mg L−1 level). These findings reveal the significance of surface defects of Mg(OH)2 for extracting As(V) and Cr(VI) from low-concentration solutions. The proposed strategy of surface defect-fabrication should provide a useful insight into designing effective adsorbents for the selective extraction of heavy metal oxyanions at low concentration.


 Please wait while we load your content...
Please wait while we load your content...