Influence of organic compounds on the sulfidation of copper oxide nanoparticles†
Abstract
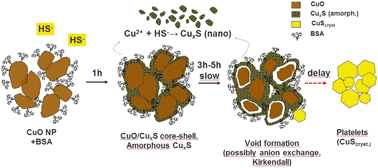
Dissolved organic matter (DOM) influences the colloidal properties of engineered nanomaterials (ENMs) and impacts their transport and transformation in the environment. Sulfidation affects the fate and the ecological effects of released chalcophile ENMs. For copper oxide nanoparticles (CuO-NPs), the sulfidation and its kinetics have been described. However, the influence of DOM on this reaction has not been investigated yet. In wastewater systems, having high bisulfide contents, the DOM mainly consists of proteins, polysaccharides and humic substances. We therefore investigated the influence of the organic compounds bovine serum albumin (BSA, model protein), alginate (ALG, model polysaccharide), and polyacrylic acid (PAA, natural organic matter analogue) as well as that of natural humic acid (HA) and filtered wastewater (FW) on the sulfidation of CuO-NPs. Experiments were conducted in solutions buffered to pH 8.0 at concentrations of 1.3 mM CuO and ∼3.2 mM HS−. Increasing amounts of organic compounds were added to reach concentrations of 10–1000 mg L−1. Reacted CuO-NPs were collected at selected time points and characterized using Cu K-edge X-ray absorption spectroscopy and analytical electron microscopy. The sulfidation of the CuO-NPs was complete after 3 h in all experiments, except in the presence of 1000 mg L−1 BSA where ∼30% of CuO remained untransformed. Complete sulfidation of the CuO-NPs as observed in experiments conducted in FW suggests quantitative sulfidation of CuO-NPs in real wastewater. Without organic compounds, initially formed amorphous CuxS transformed into covellite over time. The presence of BSA, HA, ALG, and FW (but not PAA) hampered this transformation resulting in higher fractions of CuxS after sulfidation. Different amounts of CuxS with sizes of 10 kDa up to 10 nm formed during sulfidation depending on the type of organic compound. The properties of the CuxS-NPs formed by CuO-NP sulfidation must be considered when assessing their environmental impact.


 Please wait while we load your content...
Please wait while we load your content...