Theoretical insight into the adsorption of aromatic compounds on graphene oxide†
Abstract
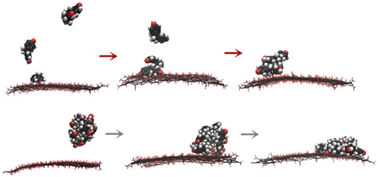
In this work, adsorption of aromatic compounds (ACs) on graphene oxide (GO) was systematically investigated. Bisphenol A, nitrobenzene, phenol, benzoic acid, and salicylic acid were employed as representatives of ACs. Experimental isotherm analysis indicated that the order of adsorption capacity is nitrobenzene > BPA > phenol > salicylic acid > benzoic acid. To examine which mechanism (including π–π, hydrogen bond, vdW, and hydrophobic interactions) governed the adsorption capacity, the π-stacking ability, hydrogen bond interaction energy, polarizability, and interaction intensity of ACs with water were examined using molecular dynamics simulations and density functional theory calculations. The results showed that the adsorption capacity was mainly guided by the π-stacking ability of ACs. Hydrophobic, GO–AC hydrogen bond, van der Waals, and electrostatic interactions may contribute to the adsorption of ACs on GO, but are not important in regulating the adsorption capacity. Local configurations of ACs adsorbed on GO were captured, and two patterns for multilayer adsorption were observed. Further analysis suggested that upon adsorbing on GO, the translational motion of ACs in water will be suppressed; however, the solvent accessible surface area will be increased, which may increase the bio-accessibility of ACs.


 Please wait while we load your content...
Please wait while we load your content...
