Reversible Fe(ii) uptake/release by magnetite nanoparticles†
Abstract
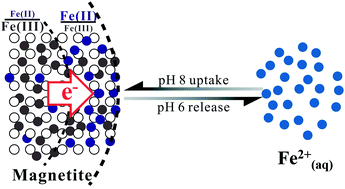
Magnetite commonly coexists with aqueous Fe2+ (Fe2+(aq)) in anoxic subsurface environments. Complex interactions between magnetite and Fe2+(aq) profoundly impact redox potential fluctuations in surrounding environment and biogeochemical cycles of important elements and contaminants. However, the ability of magnetite to act as a source/sink of electron equivalents through fluctuations in solution pH or the activity of Fe2+(aq) remains poorly quantified. We systematically studied the interrelationships between equilibrium Fe2+(aq) concentrations and structural versus surface-localized Fe(II)/Fe(III) ratios in magnetite using micro X-ray diffraction and synchrotron-based X-ray magnetic circular dichroism, respectively, under different controlled experimental conditions. Relative to pH 7, at pH 6 proton-promoted dissolution yields Fe2+(aq) release from magnetite nanoparticles, coupled to a decrease in the structural Fe(II)/Fe(III) ratio by electron hopping along the octahedral sublattice from the particle interior to the surface. At pH 8, magnetite sorbs Fe2+(aq), increasing both the structural and surface-localized Fe(II)/Fe(III) ratio. Amendments of Fe2+(aq) inhibit acidic Fe2+(aq) release and promote Fe2+(aq) uptake at more basic conditions, whereas increasing magnetite loading facilitates Fe2+(aq)–magnetite interaction at the same respective pH extremes. The reversible flow of Fe(II) across the magnetite–solution interface under different conditions implies that the redox reactivity of magnetite nanoparticles is quickly responsive to changes in environmental conditions, such as an increase in pH due to groundwater passing through carbonate-rich rocks, via a dynamic redistribution of electron equivalents between particle interiors and the solid/water interface.
- This article is part of the themed collection: Best Papers 2018 – Environmental Science: Nano


 Please wait while we load your content...
Please wait while we load your content...
