Quantum mechanical study on the oxidation of ethyl vinyl ketone initiated by an OH radical†
Abstract
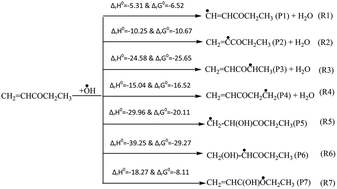
Oxidation of ethyl vinyl ketone (CH2CHCOCH2CH3) by an OH radical was carried out using the M06-2X/6-311++G(d,p) level of theory. For the OH-initiated oxidation of ethyl vinyl ketone (EVK), we have considered six H-atom abstractions and three addition reactions. From the energetic calculation of the species involved therein, the potential energy surface (PES) of all the reaction channels was constructed. From the energy profile, we found that the H-atom abstraction from the methylene group (–CH2–) of CH2CHCOCH2CH3 is energetically more favourable than the other H-abstraction channels. Moreover, we also observed that OH-addition to the α-carbon of the carbon–carbon double bond of the title molecule is energetically and thermodynamically more dominant than β-carbon and carbonyl carbon. The rate coefficients for all the reaction channels were calculated using the canonical transition state theory at the temperature range of 250–450 K and it reveals that among all the reaction channels, OH-addition to α-carbon is kinetically more dominant to the total rate constant. The total rate coefficient for the reaction at 298 K is found to be in good agreement with the reported experimental rate constant. Finally, we have determined the atmospheric lifetime of the title molecule.


 Please wait while we load your content...
Please wait while we load your content...