Development of a hydrophilic interaction liquid chromatography (HILIC) method for the chemical characterization of water-soluble isoprene epoxydiol (IEPOX)-derived secondary organic aerosol†
Abstract
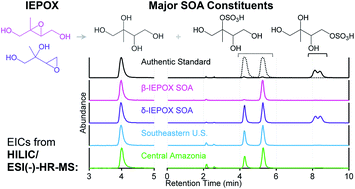
Acid-catalyzed multiphase chemistry of isoprene epoxydiols (IEPOX) on sulfate aerosol produces substantial amounts of water-soluble secondary organic aerosol (SOA) constituents, including 2-methyltetrols, methyltetrol sulfates, and oligomers thereof in atmospheric fine particulate matter (PM2.5). These constituents have commonly been measured by gas chromatography interfaced to electron ionization mass spectrometry (GC/EI-MS) with prior derivatization or by reverse-phase liquid chromatography interfaced to electrospray ionization high-resolution mass spectrometry (RPLC/ESI-HR-MS). However, both techniques have limitations in explicitly resolving and quantifying polar SOA constituents due either to thermal degradation or poor separation. With authentic 2-methyltetrol and methyltetrol sulfate standards synthesized in-house, we developed a hydrophilic interaction liquid chromatography (HILIC)/ESI-HR-quadrupole time-of-flight mass spectrometry (QTOFMS) protocol that can chromatographically resolve and accurately measure the major IEPOX-derived SOA constituents in both laboratory-generated SOA and atmospheric PM2.5. 2-Methyltetrols were simultaneously resolved along with 4–6 diastereomers of methyltetrol sulfate, allowing efficient quantification of both major classes of SOA constituents by a single non-thermal analytical method. The sum of 2-methyltetrols and methyltetrol sulfates accounted for approximately 92%, 62%, and 21% of the laboratory-generated β-IEPOX aerosol mass, laboratory-generated δ-IEPOX aerosol mass, and organic aerosol mass in the southeastern U.S., respectively, where the mass concentration of methyltetrol sulfates was 171–271% the mass concentration of methyltetrol. Mass concentrations of methyltetrol sulfates were 0.39 and 2.33 μg m−3 in a PM2.5 sample collected from central Amazonia and the southeastern U.S., respectively. The improved resolution clearly reveals isomeric patterns specific to methyltetrol sulfates from acid-catalyzed multiphase chemistry of β- and δ-IEPOX. We also demonstrate that conventional GC/EI-MS analyses overestimate 2-methyltetrols by up to 188%, resulting (in part) from the thermal degradation of methyltetrol sulfates. Lastly, C5-alkene triols and 3-methyltetrahydrofuran-3,4-diols are found to be largely GC/EI-MS artifacts formed from thermal degradation of 2-methyltetrol sulfates and 3-methyletrol sulfates, respectively, and are not detected with HILIC/ESI-HR-QTOFMS.
- This article is part of the themed collection: Atmospheric Surfaces


 Please wait while we load your content...
Please wait while we load your content...
