Active iron bound to SOM catalyzes the oxidization of alkanes in soil by H2O2†
Abstract
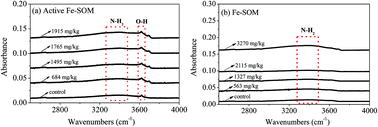
To explore the oxidation effects and mechanisms for the oxidation of alkanes by H2O2 in a Fenton system catalyzed by two types of iron bound to soil organic matter (Fe-SOM) in crude oil-contaminated soil, an oxidation experiment was performed in active Fe-SOM and Fe-SOM systems. The results showed that the TPH removal ability of active Fe-SOM (average 0.36 g TPH/g Fe-SOM) was 2.25-fold higher than the corresponding value of Fe-SOM. Active Fe-SOM contained both –NH2 and –OH functional groups, and had a higher content of iron with high binding energy, while Fe-SOM only contained –NH2 groups. Thus, a large yield of hydroxyl radicals (·OH) was generated (8.92 a.u.) by active Fe-SOM catalyzing the decomposition of H2O2, while the corresponding yield of ·OH in the Fe-SOM system was only 4.81 a.u. In addition, the removal efficiency of C17–C23 (70%) was comparable to that of C24–C30 (69%), not restricted by the hydrophobicity of different alkanes. The alkane removal by active Fe-SOM was higher than that by Fe-SOM, although the content of Fe-SOM was double that of active Fe-SOM. In summary, the active Fe-SOM formed in the soil sample containing humic acid-like and hydrophobic acid derivates could catalyze H2O2 decomposition to improve the removal efficiency of crude oil in contaminated soil.


 Please wait while we load your content...
Please wait while we load your content...