Characterization of manganese oxide amendments for in situ remediation of mercury-contaminated sediments†
Abstract
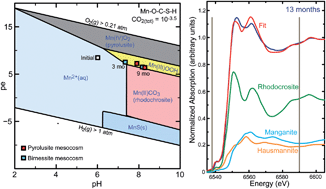
Addition of Mn(IV)-oxide phases pyrolusite or birnessite was investigated as a remedial amendment for Hg-contaminated sediments. Because inorganic Hg methylation is a byproduct of bacterial sulfate reduction, reaction of Mn(IV) oxide with pore water should poise sediment oxidation potential at a level higher than favorable for Hg methylation. Changes in Mn(IV)-oxide mineralogy and oxidation state over time were investigated in sediment tank mesocosm experiments in which Mn(IV)-oxide amendment was either mixed into Hg-contaminated sediment or applied as a thin-layer sand cap on top of sediment. Mesocosms were sampled between 4 and 15 months of operation and solid phases were characterized by X-ray absorption spectroscopy (XAS). For pyrolusite-amended sediments, Mn(IV) oxide was altered to a mixture of Mn(III)-oxyhydroxide and Mn, Fe(III, II)-oxide phases, with a progressive increase in the Mn(II)-carbonate fraction over time as mesocosm sediments became more reduced. For birnessite-amended sediments, both Mn(III) oxyhydroxide and Mn(II) carbonate were identified at 4 months, indicating a faster rate of Mn reduction compared to pyrolusite. After 15 months of reaction, birnessite was converted completely to Mn(II) carbonate, whereas residual Mn, Fe(III, II)-oxide phases were still present in addition to Mn(II) carbonate in the pyrolusite mesocosm. In the thin-layer sand cap mesocosms, no changes in either pyrolusite or birnessite XAS spectra were observed after 10 months of reaction. Equilibrium phase relationships support the interpretation of mineral redox buffering by mixed-valent (Mn, Fe)(III, II)-oxide phases. Results suggest that amendment longevity for redox buffering can be controlled by adjusting the mass and type of Mn(IV) oxide applied, mineral crystallinity, surface area, and particle size. For a given site, amendment capping versus mixing with sediment should be evaluated to determine the optimum treatment approach, which may vary depending on application constraints, rate of Mn(IV) oxide transformation, and frequency of reapplication to maintain desired oxidation state and pH.
- This article is part of the themed collection: Mercury Biogeochemistry, Exposure, and Impacts


 Please wait while we load your content...
Please wait while we load your content...