Physiological modes of action across species and toxicants: the key to predictive ecotoxicology†
Abstract
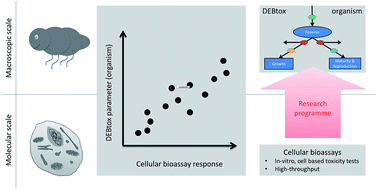
As ecotoxicologists we strive for a better understanding of how chemicals affect our environment. Humanity needs tools to identify those combinations of man-made chemicals and organisms most likely to cause problems. In other words: which of the millions of species are at risk from pollution? And which of the tens of thousands of chemicals contribute most to the risk? We identified our poor knowledge on physiological modes of action (how a chemical affects the energy allocation in an organism), and how they vary across species and toxicants, as a major knowledge gap. We also find that the key to predictive ecotoxicology is the systematic, rigorous characterization of physiological modes of action because that will enable more powerful in vitro to in vivo toxicity extrapolation and in silico ecotoxicology. In the near future, we expect a step change in our ability to study physiological modes of action by improved, and partially automated, experimental methods. Once we have populated the matrix of species and toxicants with sufficient physiological mode of action data we can look for patterns, and from those patterns infer general rules, theory and models.
- This article is part of the themed collections: Best Papers 2018 – Environmental Science: Processes & Impacts, Best Papers from 2018 in the Environmental Science Family of Journals, Editors Choice: Planetary Health and Modeling in Environmental Chemistry


 Please wait while we load your content...
Please wait while we load your content...