Sequential catalysis controls selectivity in electrochemical CO2 reduction on Cu†
Abstract
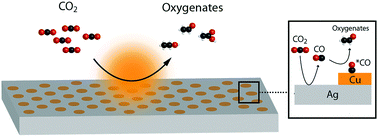
Electrochemical reduction of CO2 in aqueous media is a strategy for sustainable production of fuels and commodity chemicals. Cu is the only catalyst which converts CO2 to significant quantities of hydrocarbons and oxygenates. Here we demonstrate that oxygenate products can be favored over hydrocarbons by positioning a local source of CO generated by a CO producing catalyst (Au or Ag) in close proximity to a Cu catalyst. Use of a bimetallic device comprising interdigitated and independently controllable lines of Au and Cu allows the local CO concentration to be modulated. Notably, diffusional simulations show that the saturation concentration of CO can be exceeded locally. Actuating both the Au and Cu lines increases the oxygenate to ethylene ratio compared to actuating Cu only. Increasing the relative area of CO-producing Au relative to Cu also increases this ratio. These insights are translated into a second bimetallic system comprising Cu dots/lines patterned directly onto a Ag substrate, allowing for the distance between Cu and the CO generating metal to be precisely controlled. Controlling the relative areas of Ag and Cu allows for tuning of the oxygenate to ethylene ratio from 0.59 to 2.39 and an increase in oxygenate faradaic efficiency from 21.4% to 41.4%, while maintaining the selectivity to C2/C3 products in the 50–65% range. We attribute this change in selectivity to be due to an increased *CO coverage on Cu. By utilizing diffusional transport of CO to the Cu, a sequential catalysis pathway is created which allows for the control of oxygenate selectivity in aqueous CO2 reduction.


 Please wait while we load your content...
Please wait while we load your content...