Abstract
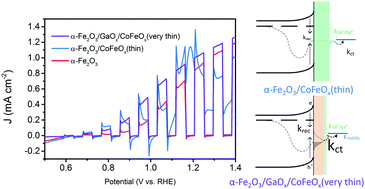
Photoelectrochemical solar water splitting into hydrogen and oxygen offers an elegant and potentially efficient way to store solar energy in the chemical bonds of hydrogen, but the oxygen evolution rate is quite limited. The deposition of an oxygen evolution catalyst on the photoanode can enhance oxygen evolution, although the precise interplay between the semiconductor and the catalyst remains poorly understood and unoptimized. In this work, we use a combination of electrochemical approaches, including photoelectrochemical impedance spectroscopy and intensity modulated photocurrent spectroscopy, to unravel the nature of the interactions between different loadings of an electrocatalyst (CoFeOx) and a hematite (α-Fe2O3) semiconductor. A thin layer of CoFeOx mainly reduces surface charge recombination, while an extremely thin layer enhances charge transfer kinetics. Moreover, an interlayer of GaOx modifies the surface state distribution and increases the charge transfer rate even further. These findings point to new opportunities for understanding and manipulating complex photoanodes for oxygen evolution.


 Please wait while we load your content...
Please wait while we load your content...