Selective CO2 reduction to C3 and C4 oxyhydrocarbons on nickel phosphides at overpotentials as low as 10 mV†
Abstract
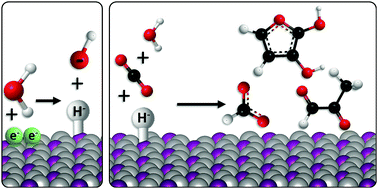
We introduce five nickel phosphide compounds as electro-catalysts for the reduction of carbon dioxide in aqueous solution, that achieve unprecedented selectivity to C3 and C4 products (the first such report). Three products: formic acid (C1), methylglyoxal (C3), and 2,3-furandiol (C4), are observed at potentials as low as +50 mV vs. RHE, and at the highest half-reaction energy efficiencies reported to date for any >C1 product (99%). The maximum selectivity for 2,3-furandiol is 71% (faradaic efficiency) at 0.00 V vs. RHE on Ni2P, which is equivalent to an overpotential of 10 mV, with the balance forming methylglyoxal, the proposed reaction intermediate. P content in the series correlates closely with both the total C products and product selectivity, establishing definitive structure–function relationships. We propose a reaction mechanism for the formation of multi-carbon products, involving hydride transfer as the potential-determining step to oxygen-bound intermediates. This unlocks a new and more energy-efficient reduction route that has only been previously observed in nickel-based enzymes. This performance contrasts with simple metallic catalysts that have poor selectivity between multi-carbon products, and which require high overpotentials (>700 mV) to achieve comparable reaction rates.


 Please wait while we load your content...
Please wait while we load your content...