Water splitting by electrolysis at high current densities under 1.6 volts†
Abstract
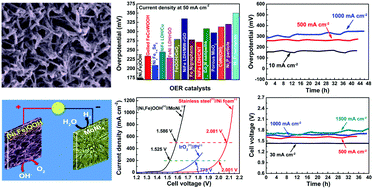
Splitting water into hydrogen and oxygen by electrolysis using electricity from intermittent waste heat, wind, or solar energies is one of the easiest and cleanest methods for high-purity hydrogen production and an effective way to store the excess electrical power. The key dilemma for efficient large-scale production of hydrogen by splitting of water via the hydrogen and oxygen evolution reactions (HER and OER, respectively) is the high overpotential required, especially for the OER. We report an exceptionally active and durable OER catalyst yielding current densities of 500 and 1000 mA cm−2 at overpotentials of only 259 mV and 289 mV in alkaline electrolyte, respectively, fulfilling the commercial criteria of the OER process. Together with a good HER catalyst, we have achieved the commercially required current densities of 500 and 1000 mA cm−2 at 1.586 and 1.657 V, respectively, with very good stability, dramatically lower than any previously reported voltage. This discovery sets the stage for large-scale hydrogen production by water splitting using excess electrical power whenever and wherever available.
- This article is part of the themed collection: 2018 Energy and Environmental Science HOT Articles


 Please wait while we load your content...
Please wait while we load your content...