Ultra-rapid rates of water splitting for biohydrogen gas production through in vitro artificial enzymatic pathways†
Abstract
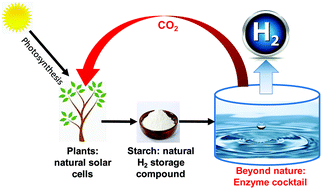
Unlocking the potential of the hydrogen economy requires breakthroughs in production, storage, distribution, and infrastructure. Here we demonstrate an in vitro artificial enzymatic pathway that can produce hydrogen at extremely high rates by splitting water energized by carbohydrates (e.g., starch). This fifteen hyperthermophilic enzymes pathway comprises ATP-free starch phosphorylation, an NAD-based pentose phosphate pathway, and a biomimetic electron transport chain consisting of a diaphorase, an electron mediator benzyl viologen (BV), and a [NiFe]-hydrogenase, whereas fast electron transfer was facilitated by utilizing a BV-conjugated diaphorase. The highest reaction rate of 530 mmol H2 per L per h was accomplished at 80 °C. Two NAD-conjugated dehydrogenases were further applied to enable nine-day hydrogen production with a total turnover number of NAD of over 100 000, along with hyperthermophilic enzymes. This biohydrogen production system characterized by the highest chemical-energy efficiency and an exceptionally-high reaction rate addresses challenges associated with cost-effective, distributed hydrogen production, off-board hydrogen storage, and infrastructure.


 Please wait while we load your content...
Please wait while we load your content...