Empowering multicomponent cathode materials for sodium ion batteries by exploring three-dimensional compositional heterogeneities†
Abstract
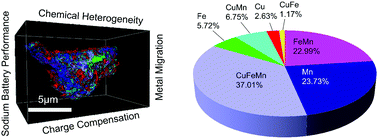
Affordable sodium ion batteries hold great promise for revolutionizing stationary energy storage technologies. Sodium layered cathode materials are usually multicomponent transition metal (TM) oxides and each TM plays a unique role in the operating cathode chemistry, e.g., redox activity, structural stabilization. Engineering the three-dimensional (3D) distribution of TM cations in individual cathode particles can take advantage of a depth-dependent charging mechanism and enable a path towards tuning local TM–O chemical environments and building resilience against cathode–electrolyte interfacial reactions that are responsible for capacity fading, voltage decay and safety hazards. In this study, we create 3D compositional heterogeneity in a ternary and biphasic (O3–P3) sodium layered cathode material (Na0.9Cu0.2Fe0.28Mn0.52O2). The cells containing this material deliver stable voltage profiles, and discharge capacities of 125 mA h g−1 at C/10 with almost no capacity fading after 100 cycles and 75 mA h g−1 at 1C with negligible capacity fading after 200 cycles. The direct performance comparison shows that this material outperforms other materials with similar global compositions but different mesoscale chemical distributions. Synchrotron X-ray spectroscopy/imaging and density functional theory studies reveal depth-dependent chemical environments due to changes to factors such as charge compensation and strength of orbital hybridization. Finally, 3D spectroscopic tomography illuminates the path towards optimizing multicomponent sodium layered cathode materials, to prevent the migration of TMs upon prolonged cycling. The study reports an inaugural effort of multifaceted and counterintuitive investigation of sodium layered cathode materials and strongly implies that there is plenty of room at the bottom by tuning nano/meso scale chemical distributions for stable cathode chemistry.


 Please wait while we load your content...
Please wait while we load your content...