The use of poly-cation oxides to lower the temperature of two-step thermochemical water splitting†
Abstract
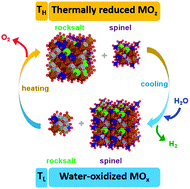
We report the discovery of a new class of oxides – poly-cation oxides (PCOs) – that consist of multiple cations and can thermochemically split water in a two-step cycle to produce hydrogen (H2) and oxygen (O2). Specifically, we demonstrate H2 yields of 10.1 ± 0.5 mL-H2 per g and 1.4 ± 0.5 mL-H2 per g from (FeMgCoNi)Ox (x ≈ 1.2) with thermal reduction temperatures of 1300 °C and 1100 °C, respectively, and also with background H2 during the water splitting step. Remarkably, these capacities are mostly higher than those from measurements and thermodynamic analysis of state-of-the-art materials such as (substituted) ceria and spinel ferrites. Such high-performance two-step cycles ≤1100 °C are practically relevant for today's chemical infrastructure at large scale, which relies almost exclusively on thermochemical transformations in this temperature regime. It is likely that PCOs with complex cation compositions will offer new opportunities for both fundamental investigations of redox thermochemistry as well as scalable H2 production using infrastructure-compatible chemical systems.
- This article is part of the themed collection: 2018 Energy and Environmental Science HOT Articles


 Please wait while we load your content...
Please wait while we load your content...