Light-assisted electrochemical water-splitting at very low bias voltage using metal-free polythiophene as photocathode at high pH in a full-cell setup†
Abstract
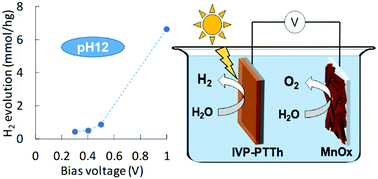
We report the use of polyterthiophenes (PTTh) as a rare, combined light-harvester and catalyst for the hydrogen evolution reaction at high pH. Illuminated PTTh in combination with a MnOx anode in a two-electrode setup exhibited stable water-splitting at a bias-potential of only 0.3 V at pH 12. This confirms that PTTh used as photocathode can produce photovoltages of at least 900 mV for the hydrogen evolution reaction at high pH and is therefore a suitable match for traditional water-oxidation catalysts such as MnOx, CoOx, and RuOx. Films of PTTh were fabricated using a novel, metal-free method where iodine vapor is used as oxidant; thus, previously observed interference from metal residuals was omitted and thereby a gravimetric photo electrocatalytic conversion rate of 330 mmol(H2) h−1 g−1 was achieved in phosphate buffer (pH 7) at 0 V vs. RHE. A striking property of this new strand of polythiophene was an “inverse” correlation (compared to that predicted using the Nernstian relationship) between the onset potential for the light-assisted water reduction reaction and pH: the potential (vs. RHE) required for a given current was more positive at higher pH, which is not observed for other (photo-) electrocatalytic materials, and indicates that the charge neutralization of PTTh (rather than the pH) guides the onset potential.


 Please wait while we load your content...
Please wait while we load your content...