Li2CO3-free Li–O2/CO2 battery with peroxide discharge product†
Abstract
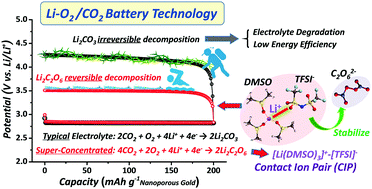
Owing to the exceptionally high energy potential of lithium–oxygen chemistry, lithium–air batteries have received much attention as appealing alternatives to current state-of-art lithium ion batteries. However, owing to the presence of non-O2 components in ambient air, the practical performance of Li–air batteries is limited to only a few cycles with low energy efficiency. In particular, when CO2 is incorporated into the battery system, Li2CO3 is formed during discharging, which results in serious climbing of the charge potential and subsequent decomposition of the cell components. Herein, by introducing an appropriate electrolyte into an aggregated contact ion pair (CIP) structure, we demonstrate a Li–O2/CO2 battery, via formation of stabilized peroxodicarbonate (C2O62−) rather than Li2CO3 in the electrolyte, that operates with a very low charge potential (3.5 V). We anticipate that this discovery will provide a rational design strategy for modifying the reaction pathway of Li–O2/CO2 battery and accelerating the evolution of Li–O2 batteries to Li–air batteries.


 Please wait while we load your content...
Please wait while we load your content...