Gold plasmon-induced photocatalytic dehydrogenative coupling of methane to ethane on polar oxide surfaces†
Abstract
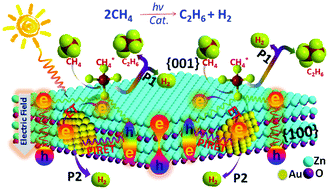
This work shows the solar-driven dehydrogenative coupling of methane to ethane at room temperature. A solar-to-C2H6 energy conversion efficiency of 0.08% was achieved on the polar surface of Au/ZnO porous nanosheets (NSs), where methane C–H bonds are polarized and dissociated by the local electric field normal to the polar {001} plane, and finally converted into ethane and hydrogen through a radical coupling pathway. Mechanistic studies suggest that the Au-plasmon-induced resonance energy transfer modulates charge carrier energetics to trigger the stoichiometric conversion. Hot electrons reduce protons to H2, which is the rate-determining step of methane coupling.


 Please wait while we load your content...
Please wait while we load your content...