Straightforward formation of carbocations from tertiary carboxylic acids via CO release at room temperature†
Abstract
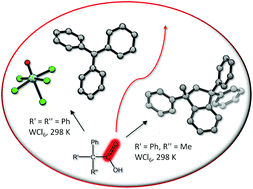
We report an unprecedented mode of reactivity of carboxylic acids. A series of tertiary carboxylic acids, containing at least one phenyl α-substituent, undergo loss of carbon monoxide at room temperature (295 K), by a one pot reaction with 0.5–1 molar equivalents of WCl6 in dichloromethane. A plausible mechanism for the Ph3CCO2H/WCl6 reaction, leading to [CPh3][WOCl5] and Ph3CCl, is proposed on the basis of DFT calculations. The analogous reactions involving CEt(Ph)2CO2H, CMe(Ph)2CO2H and CMe2(Ph)CO2H selectively afforded stable hydrocarbons (alkene or indene, depending on the case), apparently resulting from the rearrangement of elusive tertiary carbocations.


 Please wait while we load your content...
Please wait while we load your content...