Modifying methylalumoxane via alkyl exchange†
Abstract
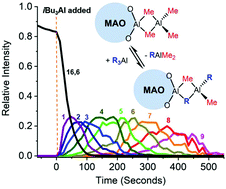
Methylalumoxane (MAO) ionizes highly selectively in the presence of octamethyltrisiloxane (OMTS) to generate [Me2Al·OMTS]+ [(MeAlO)16(Me3Al)6Me]−. We can take advantage of this transformation to examine the reactivity of a key component of MAO using electrospray ionization mass spectrometry (ESI-MS), and here we describe the reactivity of this pair of ions with other trialkyl aluminum (R3Al) components. Using continuous injection methods, we found Et3Al to exchange much faster and extensively at room temperature in fluorobenzene (t½ ∼2 s, up to 25 exchanges of Me for Et) than iBu3Al (t½ ∼40 s, up to 11 exchanges) or Oct3Al (t½ ∼200 s, up to 7 exchanges). The exchanges are reversible and the methyl groups on the cation are also observed to exchange with the added R3Al species. These results point to the reactive components of MAO having a structure that deviates significantly from the cage-like motifs studied to date.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...