Theoretical studies on Rh(iii)-catalyzed regioselective C–H bond cyanation of indole and indoline†
Abstract
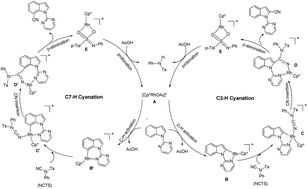
Density functional theory calculations were carried out to study the reaction mechanism of the Rh(III)-catalyzed regioselective C–H cyanation of indole and indoline with N-cyano-N-phenyl-para-methylbenzenesulfonamide (NCTS). This mechanism involves four major steps: C–H activation, cyano group insertion, β-N elimination, and regeneration of active species. How different indole and indoline substrates affect the regioselectivity of C–H bond cyanation has been examined and analyzed in detail. Our calculation results indicate that the regioselectivity of C–H bond cyanation of indole depends on the nucleophilicity of carbon atoms in C–Rh(III) bonds to the cyano group. For indoline, it can be attributed to the different hybridization platforms of the C–H bond activation.


 Please wait while we load your content...
Please wait while we load your content...