Hydroboration of carbon dioxide with catechol- and pinacolborane using an Ir–CNP* pincer complex. Water influence on the catalytic activity†‡
Abstract
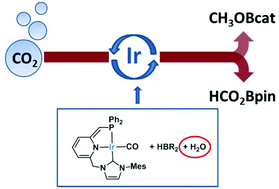
Iridium complexes based on deprotonated lutidine-derived CNP* pincers 2a/2b selectively catalyzed the hydroboration of CO2 under mild conditions (1–2 bar CO2, 30 °C) to methoxyborane using HBcat (TOF up to 56 h−1) and to the formate level with HBpin (TOF up to 1245 h−1). Interestingly, an intriguing, positive water effect on the reaction rates has been observed. NMR spectroscopy and ESI-MS analysis of the hydroboration reactions have shown the formation of ligand-protonated [Ir(CNP)(CO)(BR2)H][B(R2)2] (R2 = catecholate, pinacolate) derivatives under catalytic conditions. Control experiments, however, have demonstrated that these derivatives are not catalytically competent species in the hydroboration of CO2.


 Please wait while we load your content...
Please wait while we load your content...