Disorder induced polymorphic transitions in the high hydrogen density compound Sr(BH4)2(NH3BH3)2†
Abstract
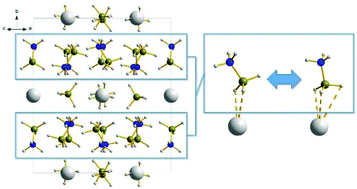
The new compound Sr(BH4)2(NH3BH3)2 has been synthesized and characterized with in situ powder X-ray diffraction and fast (28 or 60 kHz) magic angle spinning 1H, 11B and 15N NMR and structurally optimized with density functional theory calculations. This investigation reveals complex structural rearrangements for this compound as a function of temperature. A room temperature orthorhombic polymorph, α-Sr(BH4)2(NH3BH3)2, with the space group symmetry Pbca, has been determined with a layered structure of alternating ammonia borane and Sr(BH4)2, partially stabilized by dihydrogen bonding. Surprisingly the crystal symmetry is lowered upon heating, as evidenced both by in situ synchrotron powder X-ray diffraction and 11B MAS NMR data, resulting in an intermediate polymorph, β′-Sr(BH4)2(NH3BH3)2, present from ∼65 to 115 °C. β-Sr(BH4)2(NH3BH3)2, a sub structure of the β′-polymorph showing higher symmetry with the space group symmetry Aba2, forms upon further heating. Ab initio molecular dynamics simulations show that the ammonia borane molecule can dynamically alternate between a bidentate and a tridentate coordination to Sr at finite temperature. The dynamic properties of the ammonia borane molecule in the solid state are suggested to cause the observed structural complexity. Based on simultaneous thermogravimetric analysis, differential scanning calorimetry and mass spectrometry, the decomposition of the compound was investigated showing a stabilization of ammonia borane in the structure relative to other metal borohydride ammonia boranes and neat ammonia borane.


 Please wait while we load your content...
Please wait while we load your content...