Vanadyl sulfates: molecular structure, magnetism and electrochemical activity†
Abstract
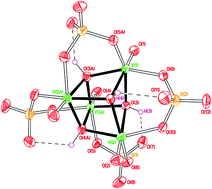
Reaction of differing amounts of vanadyl sulfate with p-tert-butylthiacalix[4]areneH4 and base allows access to the vanadyl-sulfate species [NEt4]4[(VO)4(μ3-OH)4(SO4)4]·½H2O (1), [HNEt3]5[(VO)5(μ3-O)4(SO4)4]·4MeCN (2·4MeCN) and [NEt4]2[(VO)6(O)2(SO4)4(OMe)(OH2)]·MeCN (3·MeCN). Similar use of p-tert-butylsulfonylcalix[4]areneH4, p-tert-butylcalix[8]areneH8 or p-tert-butylhexahomotrioxacalix[3]areneH3 led to the isolation of [HNEt3]2[H2NEt2]2{[VO(OMe)]2p-tert-butylcalix[8-SO2]areneH2} (4), [HNEt3]2[V(O)2p-tert-butylcalix[8]areneH5] (5) and [HNEt3]2[VIV2VV4O11(OMe)8] (6), respectively. Dc magnetic susceptibility measurements were performed on powdered microcrystalline samples of 1–3 in the T = 300–2 K temperature range. Preliminary screening for electrochemical water oxidation revealed some activity for 2 with turnover frequency (TOF) and number (TON) of 2.2 × 10−4 s−1 and 6.44 × 10−6 (mmol O2/mmol cat.), respectively. The compound 3 showed an improved electrochemical activity in the presence of water. This is related to the increased number and the rate of electrons exchanged during oxidation of V4+ species, facilitated by protons generated in the water discharge process.


 Please wait while we load your content...
Please wait while we load your content...