Single molecule magnetic behaviour in lanthanide naphthalenesulfonate complexes†
Abstract
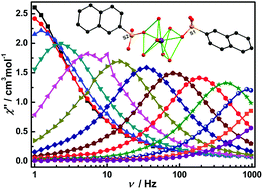
The use of 2-naphthalenesulfonate (NAS) ligand in lanthanide chemistry afforded a family of isostructural mononuclear lanthanide complexes with the formula [Ln(NAS)2(H2O)6](NAS)·3H2O [Ln = Tb (1), Dy (2), Er (3), Yb (4)]. Crystallographic studies determined a square antiprismatic geometry (D4d) for the Ln centre and crystallization in an unprecedented chiral space group. The latter was further confirmed by the observation of Cotton effects in single crystal circular dichroism (CD) spectra. Static and dynamic magnetic measurements identified weak intermolecular dipolar interactions in 2, and such effects can be waived by dilution, which was noted by the detection of zero-field single molecule magnet (SMM) behaviour and hysteresis loop in a magnetically diluted sample (5). Compounds 2–4 exhibit SMM behaviours with energy barriers of 53, 32, and 45 K, respectively. To the best of our knowledge, these complexes are the first examples of pure 4f sulfonate-based SMMs.


 Please wait while we load your content...
Please wait while we load your content...