Photoexcited state chemistry of metal–oxygen complexes
Abstract
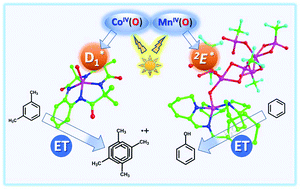
Recent advances on the excited state chemistry of metal–oxygen synthetic complexes based on earth-abundant metals such as copper, cobalt, and manganese are reviewed to show a much enhanced reactivity of the photoexcited states as compared with their relative ground states. Mononuclear copper(II)-superoxide and dinuclear copper(II)-peroxo complexes underwent copper–oxygen bond cleavage, dioxygen release, and copper(I)/dioxygen rebinding upon photoexcitation at low temperature. Photoirradiation of the cobalt–oxygen compound [(TAML)CoIV(O)]2− (6) (TAML = tetraamidomacrocyclic ligand) at 5 °C yielded a cobalt–oxygen excited state with 0.6(1) ns lifetime, showing a high reactivity in the bimolecular electron-transfer oxidations of m-xylene and anisole. An extremely long-lived excited state was generated upon photoexcitation of a manganese(IV)-oxo complex binding two Sc(OTf)3 molecules, which enabled the hydroxylation of benzene.
- This article is part of the themed collections: Inorganic chemistry approaches to saving critical elements: Replacement and 2018 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...