Comparing the reactivity of isomeric phosphinoferrocene nitrile and isocyanide in Pd(ii) complexes: synthesis of simple coordination compounds vs. preparation of P-chelated insertion products and Fischer-type carbenes†
Abstract
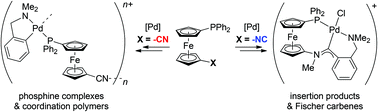
Isomeric phosphinoferrocene ligands, viz. 1′-(diphenylphosphino)-1-cyanoferrocene (1) and 1′-(diphenylphosphino)-1-isocyanoferrocene (2), show markedly different coordination behaviours. For instance, the reactions of 1 with [PdCl2(MeCN)2] and [(LNC)Pd(μ-Cl)]2 (LNC = [2-(dimethylamino-κN)methyl]phenyl-κC1) produced the “phosphine” complexes [PdCl2(1-κP)2] (7) and [(LNC)PdCl(1-κP)] (8), and the latter was converted into the coordination polymer [(LNC)Pd(μ(P,N)-1)][SbF6] (9). Conversely, the reaction of 2 with [(LNC)Pd(μ-Cl)]2 involved coordination of the phosphine moiety and simultaneous insertion of the isocyanide group into the Pd–C bond, giving rise to the P,η1-imidoyl complex [PdCl(Ph2PfcN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC6H4CH2NMe2-κ3C,N,P)] (10; fc = ferrocene-1,1′-diyl). Compound 10 was further transformed into the Fischer carbene [PdCl(Ph2PfcN(Me)CC6H4CH2NMe2-κ3P,C,N)][BF4] (11) by methylation with [Me3O][BF4]. The reactions of 2 with Pd–Me and Pd(η3-allyl) precursors also led to imidoyl complexes [Pd(μ-Cl)(Ph2PfcN
CC6H4CH2NMe2-κ3C,N,P)] (10; fc = ferrocene-1,1′-diyl). Compound 10 was further transformed into the Fischer carbene [PdCl(Ph2PfcN(Me)CC6H4CH2NMe2-κ3P,C,N)][BF4] (11) by methylation with [Me3O][BF4]. The reactions of 2 with Pd–Me and Pd(η3-allyl) precursors also led to imidoyl complexes [Pd(μ-Cl)(Ph2PfcN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR-κ2C,P)]2 (R = Me: 12, R = allyl: 15), which were cleaved with PPh3 into the corresponding monopalladium complexes [PdCl(PPh3)(Ph2PfcN
CR-κ2C,P)]2 (R = Me: 12, R = allyl: 15), which were cleaved with PPh3 into the corresponding monopalladium complexes [PdCl(PPh3)(Ph2PfcN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR-κ2C,P)] (R = Me: 13, R = allyl: 16). The treatment of 12 and 15 with thallium(I) acetylacetonate (acac) produced [Pd(acac-O,O′)(Ph2PfcN
CR-κ2C,P)] (R = Me: 13, R = allyl: 16). The treatment of 12 and 15 with thallium(I) acetylacetonate (acac) produced [Pd(acac-O,O′)(Ph2PfcN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR-κ2C,P)] (R = Me: 17, R = allyl: 18). Through proton transfer, these complexes reacted with Ph2PCH2CO2H, ultimately producing bis-chelate complexes [Pd(Ph2PCH2CO2-κ2O,P)(Ph2PfcN
CR-κ2C,P)] (R = Me: 17, R = allyl: 18). Through proton transfer, these complexes reacted with Ph2PCH2CO2H, ultimately producing bis-chelate complexes [Pd(Ph2PCH2CO2-κ2O,P)(Ph2PfcN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR)] (R = Me: 19, R = prop-1-enyl (sic!): 20). In addition, compound 13 was converted into the P-chelated carbene [PdCl(PPh3)(Ph2PfcN(Me)CMe-κ2C,P)][BF4] (14). Compounds 10, 11, 13 and 14 were studied by cyclic voltammetry and by DFT computations.
CR)] (R = Me: 19, R = prop-1-enyl (sic!): 20). In addition, compound 13 was converted into the P-chelated carbene [PdCl(PPh3)(Ph2PfcN(Me)CMe-κ2C,P)][BF4] (14). Compounds 10, 11, 13 and 14 were studied by cyclic voltammetry and by DFT computations.


 Please wait while we load your content...
Please wait while we load your content...