Abstract
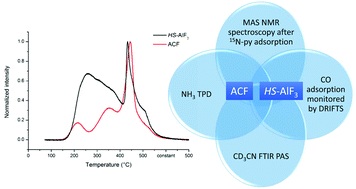
Aluminium chlorofluoride (ACF) and high-surface aluminium fluoride (HS-AlF3) were analyzed by a set of characterization methods to assess their acidic properties: NH3-TPD, CO adsorption followed by DRIFTS, CD3CN-PAS-FTIR and MAS NMR spectroscopy after 15N-pyridine adsorption. Both catalysts contain very strong and medium-strong Lewis acid sites as confirmed by CO adsorption, in which small differences arise from the morphological properties of each catalyst, with ACF being microporous and HS-AlF3 mesoporous. Shifts of the CO vibration band of up to 77 cm−1 were observed, which account for very strong Lewis acid sites. In addition, very strong Lewis acid sites could be identified by CD3CN-PAS for both catalysts, exhibiting a shift of 95 cm−1 from free nitrile, the highest ever reported for a solid Lewis acid.


 Please wait while we load your content...
Please wait while we load your content...