The coordination chemistry of lanthanide and actinide metal ions with hydroxypyridinone-based decorporation agents: orbital and density based analyses†
Abstract
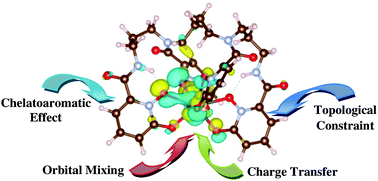
In the context of the mitigation of the biological effects of internal radionuclide contamination and for efficient decorporation, the design and development of efficient chelators for lanthanide and actinide metal ions has become a central issue. The pioneering work of Raymond and coworkers (Chem. Rev., 2003, 103, 4207–4282) led to the development of siderophore-related hydroxypyridinonate ligands for possible treatment of internalized radionuclides. However, the structure–function relationship of Ln/An bound to these ligands, particularly the bonding and coordination aspects are not clearly understood at the atomic level. Here, we have investigated the structure, binding and energetics of trivalent and tetravalent Ln/An (Sm3+, Eu3+, Am3+, Cm3+, Th4+, Pu4+) ions with spermine-based octadentate hydroxypyridinonate chelators, namely 3,4,3-LI(1,2-HOPO) and its 3,3,3 variant, using relativistic density functional theory (DFT). Furthermore, we have performed orbital and density based analyses to elucidate the nature of bonding in these complexes. In accordance with the experimental stability constant, we found the maximum binding free energy for An4+ (Pu4+, Th4+) as compared to trivalent metal ions. CDA and ECDA analyses along with orbital-based population analyses confirmed the higher ligand to metal charge transfer for An4+ than for trivalent metal ions. Furthermore, the aromaticity index analysis suggested the presence of crucial chelatoaromatic stabilization for all these metal ions with the maximum for An4+. QTAIM descriptors indicated that the binding of An/Ln with the hard oxygen donor of the ligands is of the donor–acceptor type but a higher degree of covalency exists for actinides as compared to lanthanides. Furthermore, QTAIM and molecular orbital analysis confirmed that such covalency is of the energy-driven type and strictly originates from the orbital mixing event of An-5f orbitals with the ligand orbitals.


 Please wait while we load your content...
Please wait while we load your content...