Contrasting reactivity of (boryl)(aryl)lithium-amide with electrophiles: N- vs. p-aryl-C-nucleophilic substitution†‡
Abstract
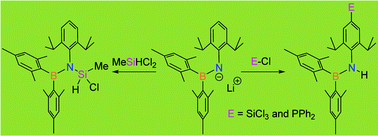
Herein we report two different reactivity modes of lithium(aryl)(boryl)amide, 4, when it is reacted with chlorosilanes such as SiCl4 and MeSiHCl2, and chlorophosphine, Ph2PCl. Thus, the reaction of lithium(aryl)(boryl)amide, 4, with MeSiHCl2 leads exclusively to an N-substitution product, 6. On the other hand, the reaction of 4 with SiCl4 and Ph2PCl proceeds completely differently affording exclusively p-aryl-C-substitution products, 5 and 7, respectively.


 Please wait while we load your content...
Please wait while we load your content...