Improving the capability of UiO-66 for Cr(vi) adsorption from aqueous solutions by introducing isonicotinate N-oxide as the functional group†
Abstract
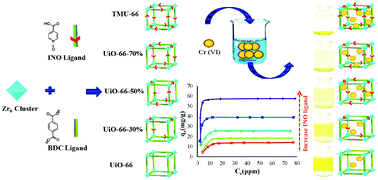
Considering the widespread production of Cr(VI) from various industrial processes and its damaging toxicity as a carcinogenic agent, it is imperative to investigate stable and efficient adsorbents with a rapid performance towards Cr(VI) adsorption. Zirconium-containing metal–organic frameworks (Zr-MOFs) have been recently used as environmentally friendly adsorbents for the reduction of metallic contaminants in aqueous media. Preparation from nontoxic metal sources, remarkable stabilities and distinguished physicochemical features, such as the high tendency of Zr-clusters to adsorb oxo-anions, beneficial structural defects, and modification of their properties via modulator synthesis, can be enumerated as the advantages of Zr-MOFs. In this study, we improved the adsorption capacity of UiO-66 as the most stable Zr-MOF for Cr(VI) adsorption from aqueous solutions through the gradual addition of an N–O functional group. This strategy ultimately led us to afford a new Zr-MOF structure (TMU-66) with a maximum Cr(VI) adsorption capacity of 60.241 mg g−1, which is 4 times the absorption capacity of UiO-66, and very fast kinetics (<3 min) that followed the pseudo-second-order kinetics. This study demonstrates that a conceptual design can be helpful in synthesizing safe and stable adsorbents with appropriate capacity and kinetics for adsorption.


 Please wait while we load your content...
Please wait while we load your content...