Photophysical properties of a D–π-A Schiff base and its applications in the detection of metal ions†
Abstract
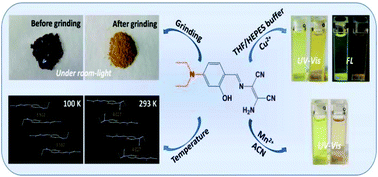
A D–π-A Schiff-base compound, 2-amino-3({[4-(diethylamino)-2-hydroxylphenyl]methylene}amino)-but-2-enedinitrile (H2L), was prepared using diaminomaleonitrile and 4-(N,N-diethylamino)salicylaldehyde. Compared with H2L at 293 K, a low temperature of 100 K makes the parallel aromatics in H2L come closer and fluorescence emission becomes weaker because of π–π interaction-caused quenching. After grinding the crystals of H2L, the colour changed from dark brown-red to yellow under room light and the fluorescence emission enhanced about 9-fold due to the damage of the intermolecular hydrogen bonds, leading to a decrease of non-radiative transition. H2L showed aggregation-induced emission enhancement (AIEE) characteristics in THF/H2O, whose mechanism is attributed to the restriction of intramolecular rotation (RIR). The UV-Vis spectra of H2L with Cu2+ in THF/H2O showed that at first a CuL complex was formed and subsequently a CuL′ complex (H2L′ = N′,N′-bis(4-N′,N′-diethylsalicylidene) ethylenediamine) was obtained. The CuL complex turned into the CuL′ complex as time prolonged. H2L acted as a dual channel chemosensor for Cu2+ ions in THF/HEPES (v/v: 2 : 8, pH = 8.0) and the CuL complex was stable in this medium. H2L is also a naked-eye probe for Mn2+ ions in CH3CN. The limits of detection are much lower than the allowable level of copper(II) and manganese(II) in drinking water set by the World Health Organization (WHO).


 Please wait while we load your content...
Please wait while we load your content...