Abstract
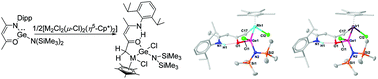
The reaction of [CH{(CMe)(2,6-iPr2C6H3N)}2]GeCl with LiN(SiMe3)2 was previously reported, which led to the formation of a hetero-fulvene type germylene, [CH{(CMe)(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(2,6-iPr2C6H3N)}2]Ge through the deprotonation of the C–H bond from the methyl substituents. In this paper, we attempted the analogous reaction with (Dipp)NCMeCHCOMeGeCl using LiN(SiMe3)2 which gave rise to a metathesis product, (Dipp)NCMeCHCOMeGeN(SiMe3)2 (2). However, the reactions of 2 with [M2Cl2(μ-Cl)2(η5-Cp*)2] (M = Rh and Ir) resulted in cyclometallated Rh and Ir complexes through the activation of the C–H bond from the germylene ligand. The complexes were characterized by single crystal X-ray analysis, which authenticated the presence of Ge–Rh and Ge–Ir bonds. DFT studies have been performed to understand the mechanism.
CH2)(2,6-iPr2C6H3N)}2]Ge through the deprotonation of the C–H bond from the methyl substituents. In this paper, we attempted the analogous reaction with (Dipp)NCMeCHCOMeGeCl using LiN(SiMe3)2 which gave rise to a metathesis product, (Dipp)NCMeCHCOMeGeN(SiMe3)2 (2). However, the reactions of 2 with [M2Cl2(μ-Cl)2(η5-Cp*)2] (M = Rh and Ir) resulted in cyclometallated Rh and Ir complexes through the activation of the C–H bond from the germylene ligand. The complexes were characterized by single crystal X-ray analysis, which authenticated the presence of Ge–Rh and Ge–Ir bonds. DFT studies have been performed to understand the mechanism.


 Please wait while we load your content...
Please wait while we load your content...