Theoretical studies on the mechanism of Pd2+-catalyzed regioselective C–H acylation of azoxybenzenes with α-oxocarboxylic acids†
Abstract
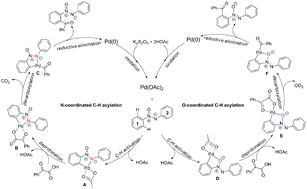
The reaction mechanism of the Pd2+-catalyzed regioselective C(sp2)–H acylation of azoxybenzenes with α-oxocarboxylic acids has been studied by density functional theory (DFT) calculations. This reaction mechanism involves five major steps: C–H activation, deprotonation, decarboxylation, reductive elimination and oxidation. Our calculation results indicate that the N-coordinated pathway is better than the O-coordinated pathway, which can be interpreted by distortion-interaction analysis of the C–H bond activation transition states. Furthermore, we also suggest that the C–H bond acylation of aryl 1 is more favorable than that of aryl 2, which can be attributed to the fact that five-membered ring transition states are more favorable than four-membered ring transition states and the ON-group has positive charge.


 Please wait while we load your content...
Please wait while we load your content...