Mechanisms of the polyol reduction of copper(ii) salts depending on the anion type and diol chain length†
Abstract
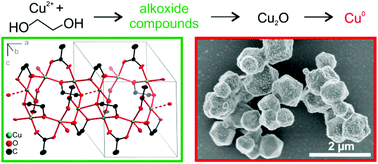
The mechanisms of the polyol reduction of copper(II) compounds were investigated by various systematic experiments. The course of the reduction in ethylene glycol strongly depends on the anion present in solution. Elemental copper can only be obtained in high yields starting from Cu(NO3)2, Cu(OAc)2, or Cu(OH)2; but not from CuCl2 or CuSO4. Intermediate compounds were observed, namely Cu2O, and the alkoxide compounds Cu(C2H4O2) and Cu3(OAc)2(C2H4O2)2. Cu3(OAc)2(C2H4O2)2 was characterised by single-crystal X-ray diffraction. It is a 2D coordination polymer with bridging bidentate acetate and bidentate deprotonated EG ligands leading to vertex- and edge-linked CuO5 polyhedra. Alkoxide compounds were also detected for higher glycol ethers. With growing glycol ether chain length the stability of alkoxide intermediates decreases, enhancing the stability range of Cu2O. Because the majority of copper species is precipitated as copper(II) alkoxide compounds or Cu2O before the reduction to elemental copper occurs, only few nuclei are formed in solution leading to microparticles instead of nanoparticles. Much smaller but strongly agglomerated particles were observed under alkaline reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...