Solvent-free thiophene-based electrolytes: synthesis of new liquid-crystalline ionic conductors for batteries: part I
Abstract
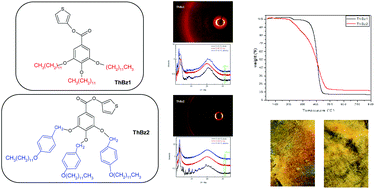
New liquid crystalline thiophene esters, thiophene-3-yl 3,4,5-tris(n-dodecan-1-yloxy)benzoate (ThBz1) and thiophene-3-yl 3,4,5-tris[4-(n-dodecan-1-yloxy)benzyloxy]benzoate (ThBz2), were synthesized for use in lithium-ion batteries as safe, new solvent-free ionic conductors. Both compounds were synthesized in multi-step reactions and were characterized via1H, 13C NMR and FT-IR spectroscopy, confirming the formation of the desired products. ThBz1 and ThBz2 showed good thermal stability and liquid crystalline behaviour. Electrochemical tests demonstrated a wide electrochemical window of 4 V for both tested ThBz compounds. Moreover, preliminary battery tests for new electrolytes in the LiCoO2/Li4Ti5O12 system confirmed their applicability in lithium-ion batteries.
- This article is part of the themed collection: Molecular metal-containing soft materials


 Please wait while we load your content...
Please wait while we load your content...