A promising PMHS/PEO blend polymer electrolyte for all-solid-state lithium ion batteries†
Abstract
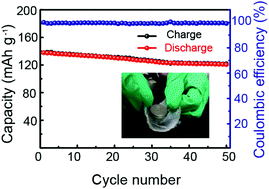
Solid-state lithium metal batteries have emerged as a promising alternative to existing liquid Li-ion batteries and can power the future storage market considering their higher energy outputs and better safety. Among various solid electrolytes, polymer electrolytes have received more attention due to their potential advantages, including wide electrochemical windows, ease of processing, low interface impedance and low cost. Polymeric electrolytes based on poly(ethylene oxide) (PEO) as a well-known polymer matrix have been extensively studied because of their highly flexible EO segments in the amorphous phase that can provide channels for lithium ion transport. However, obtaining a PEO-based solid electrolyte with high Li ion conductivity and without sacrificing mechanical strength is still a huge challenge. In this study, polymethylhydrogen-siloxane (PMHS) with low glass transition temperature and good flexibility was blended into the PEO to optimize ion transportation by the solution casting technique. The hybrid electrolyte membrane with 40% PMHS exhibited high ionic conductivity (2.0 × 10−2 S cm−1 at 80 °C), large electrochemical windows (5.2 V), a high degree of flexibility, and thermal stability. When assembling a Li/LiFePO4 battery, a reversible capacity close to 140 mA h g−1 (0.1 C) at 60 °C was delivered. In addition, a cell with this polymer electrolyte exhibits excellent stability. These results demonstrate that solid polymer electrolyte systems are eligible for next-generation high energy density all-solid-state lithium ion batteries.


 Please wait while we load your content...
Please wait while we load your content...