Spectroelectrochemical investigations of nickel cyclam indicate different reaction mechanisms for electrocatalytic CO2 and H+ reduction†
Abstract
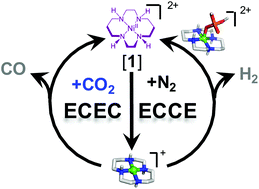
Rising levels of atmospheric carbon dioxide continue to motivate the development of catalysts that can efficiently convert CO2 to useful products in water without substantial amounts of H2 formed as a byproduct. In addition to synthetic efforts, mechanistic investigations on existing catalysts are necessary to understand the molecular factors contributing to activity and selectivity, which can guide rational improvements and increase catalyst robustness. [Ni(cyclam)]2+ (cyclam = 1,4,8,11-tetraazacyclotetradecane) is one such catalyst, known for decades to be capable of selective CO2 reduction to CO in water, but with little mechanistic information experimentally established or catalytic intermediates characterized. To better understand the mechanisms of aqueous H+ and CO2 reduction by [Ni(cyclam)]2+, spectroelectrochemical investigations were performed in conjunction with activity assays. Both large surface area glassy carbon and amorphous graphite rod working electrodes were tested, with the latter found to be significantly more active and selective for CO production. Optical, resonance Raman, and EPR spectroelectrochemical experiments on [Ni(cyclam)]2+ during catalysis under N2, CO2, and CO gases show the appearance of a single species, independent of electrode used. Identical signals are observed under oxidizing potentials. Spectroscopic and electrochemical analysis coupled with density functional theory calculations suggest that the signals observed originate from [Ni(cyclam)(H2PO4)]2+. The generation of a NiIII species under catalytic, reducing conditions suggests an ECCE mechanism for H+ reduction by [Ni(cyclam)]2+, which differs from the proton-coupled, ECEC pathway proposed for CO2 reduction. The divergent mechanisms seen for the two reactions may underlie the differential reactivity of [Ni(cyclam)]2+ towards each substrate, with implications for the design of increasingly selective molecular catalysts. This observation also highlights the substantial impact of buffer and electrode choice when characterizing and benchmarking the catalytic properties of new compounds.


 Please wait while we load your content...
Please wait while we load your content...