Abstract
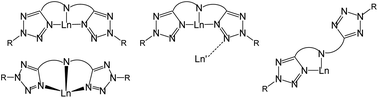
The synthesis of the protioligand 1,8-bis(2′-isopropyltetrazo-5′-yl)-3,6-di-tert-butylcarbazole, HCzTiPr2, is described. The acid–base reaction of 2 with various Ln[N(SiMe3)2]3 (Ln = Ce, Sm, Er, Yb, Y) precursors leads to products displaying a variety of different bonding modes for the CzTiPr− ligand as revealed by X-ray crystallographic studies. The smaller lanthanides form Ln(CzTiPr)[N(SiMe3)2]2 complexes (Ln = Er (3), Yb(4)) with a near planar, tridentate coordination mode for the CzTiPr− ligand. In contrast, the larger lanthanides form Ln(CzTiPr)2[N(SiMe3)2] complexes (Ln = Ce (5), Sm (6)) at room temperature that feature a sandwich structure with a highly distorted tridentate mode where tetrazole canting allows the metal to sit well out of the carbazole plane. Attempts to force additional CzTiPr− ligands onto Er or Yb at elevated temperature results in isolation of trace siloxide complexes Ln(CzTiPr)2(OSiMe3) (Ln = Er (3a), Yb (4a)) that adopt a flat tridentate ligand bonding mode similar to 3 and 4. Surprisingly, the high temperature reaction of 2 with Y[N(SiMe3)2]3 affords Y(CzTiPr)3, 7, featuring two tridentate and one bidentate CzTiPr ligands. Partial hydrolysis of 6 occurs over time to produce a hydroxo-bridged dimer that also features one bridging and one bidentate CzTiPr ligand, Sm(κ3-CzTiPr)(κ3-μ-CzTiPr)(μ-OH)2Sm(κ3-CzTiPr)(κ2-CzTiPr), 6a, further illustrating the remarkable coordinative flexibility of the CzTiPr ligand system.
- This article is part of the themed collection: In celebration of Richard Andersen’s 75th birthday


 Please wait while we load your content...
Please wait while we load your content...