Noble-metal-free Ni3N/g-C3N4 photocatalysts with enhanced hydrogen production under visible light irradiation†
Abstract
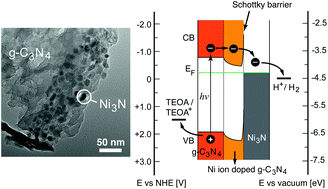
The majority of visible-light photocatalysts show very low hydrogen evolution activity in the absence of noble metal co-catalysts due to their fast carrier recombination rate. Metallic interstitial nitrides have been recognized as excellent hydrogen evolution electrocatalysts and therefore are promising alternative co-catalysts for photocatalytic hydrogen production. Herein we report a low-cost, efficient, noble-metal-free visible-light-driven hydrogen evolution photocatalyst composed of Ni3N/g-C3N4 heterostructures. The as-prepared photocatalyst with a Ni3N loading content of 3 wt% shows a high hydrogen evolution rate of 169 μmol g−1 h−1, which is slightly higher than that of 3 wt% Pt modified g-C3N4 (152.0 μmol g−1 h−1). The Ni3N/g-C3N4 photocatalysts also exhibit remarkable photostability for four consecutive cycles of photocatalytic activity tests with a total reaction time of 12 hours. The excellent performance of the Ni3N/g-C3N4 photocatalyst is ascribed to the formation of an optimal number of Ni3N/g-C3N4 heterojunctions that improve photogenerated carrier separation and offer abundant photocatalytically active sites for surface reactions.


 Please wait while we load your content...
Please wait while we load your content...